Introduction: Chronic lymphocytic leukaemia (CLL) is the most common leukaemia type. In 2020, there were an estimated 207,463 people living with CLL, with an annual incidence of approximately 4.9 per 100,000 in the United States (US). Zanubrutinib is a Bruton tyrosine kinase inhibitor that is FDA-approved and an NCCN preferred and recommended treatment of CLL. In the phase 3 ALPINE trial (NCT03734016), zanubrutinib elicited a significantly higher overall response rate and significantly longer progression-free survival (PFS) than ibrutinib. This study aimed to compare zanubrutinib versus ibrutinib in Relapsed/Refractory (R/R) CLL by calculating the number needed to treat (NNT) to avoid one progression or death and associated incremental costs.
Methods:A health-economic model was developed to evaluate the number of R/R CLL patients needed to be treated to avoid a progression or death from the US payer perspective. Payer blend was assumed with 40% Commercial and 60% Medicare payer mix. Clinical efficacy data were extracted from the ALPINE trial. Final analysis result of 24-month PFS (79.5% for zanubrutinib and 67.3% for ibrutinib) was used for the base-case analysis in the model. Treatment, adverse event management, other medical resource use, and subsequent treatment costs were considered in the model. The NNT, incremental cost per treated patient, and incremental cost per additional patient with progression or death were captured. Deterministic sensitivity analyses were conducted to assess parameter uncertainties and explore key model drivers. Scenario analyses were conducted to test the impact of different PFS estimates.
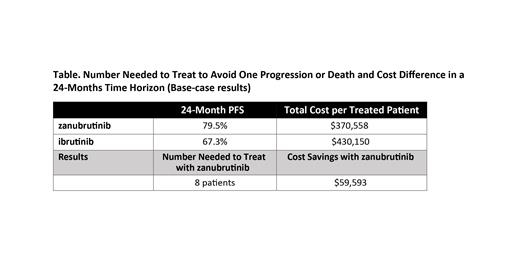
Results: The base-case results fromthe NNT model showed that for every 8 patients treated with zanubrutinib, 1 event of progression or death would be avoided compared to using ibrutinib. The total costs per patient treated with zanubrutinib and ibrutinib are $370,558 and $430,150, respectively, with a cost savings of $59,593 associated with using zanubrutinib (Table). Drug costs and PFS have the greatest impact on the incremental cost per patient. Varying the PFS scenarios, including adjustment with drug interruption, COVID death, or treatment discontinuation, change the NNT from 8 to 12, and are associated with cost savings of $58,179 to $67,153 per zanubrutinib-treated patient in a 24-month time frame. Applying the model result to a hypothetical scenario of a clinical practice of 100 patients treated with zanubrutinib versus ibrutinib suggest that approximately 13 patients will avoid disease progression events or death.
Conclusions: The NNT model suggests thatusing zanubrutinib to treat R/R CLL patients, compared to ibrutinib, will result in more favorable clinical and economic outcomes in the US.
Disclosures
Chanan-Khan:BeiGene: Consultancy, Honoraria; Ascentage: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Cellectar: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Starton: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Alpha2: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Mayo Clinic: Current Employment. Hanna:Seagen: Consultancy, Speakers Bureau; BeiGene: Consultancy, Speakers Bureau; Minnesota Oncology: Current Employment; M Health Fairview: Ended employment in the past 24 months; Bristol Myers Squibb: Speakers Bureau; G1 Therapeutics: Speakers Bureau; Exelixis: Speakers Bureau; Janssen: Speakers Bureau; Pharmacyclics: Speakers Bureau; AbbVie Inc: Speakers Bureau; Rigel Inc: Speakers Bureau; NCODA: Membership on an entity's Board of Directors or advisory committees. Xue:BeiGene USA: Current Employment. Massoudi:BeiGene, USA: Current Employment, Current equity holder in publicly-traded company. Balk:BeiGene: Current Employment, Current equity holder in publicly-traded company. ZivariPiran:CHEORS: Ended employment in the past 24 months; Evidera: Current Employment. Duan:Evidera Ltd.: Current Employment. Yang:BeiGene: Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal